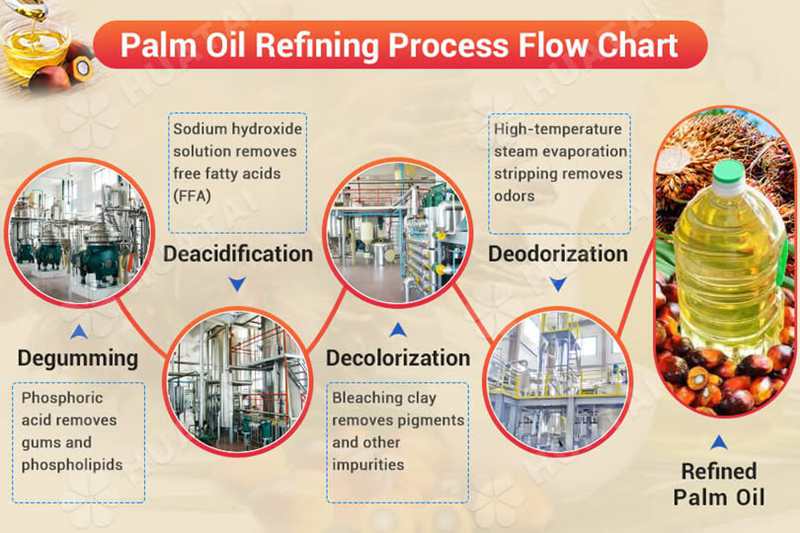
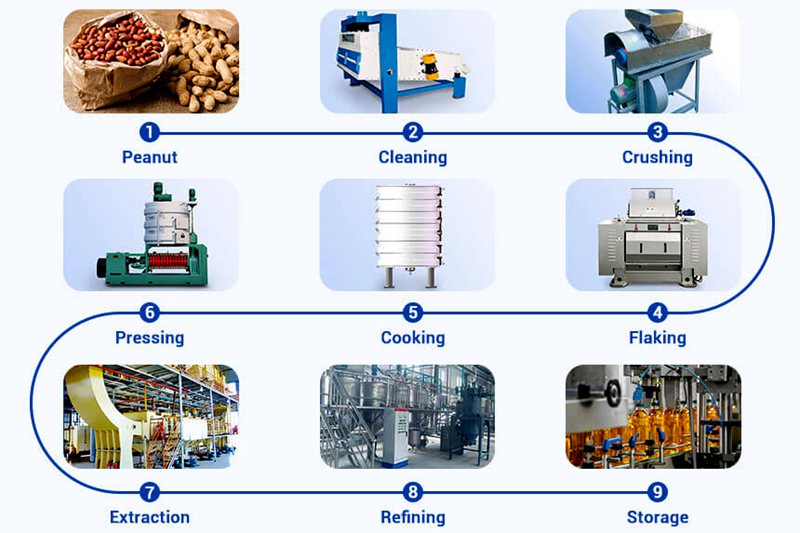
The subcritical state of a solvent is defined as the state in which it exists in a liquefied state under a certain pressure within a temperature range above its boiling point but below its critical point. Solvents used in this state, leveraging their physical properties of miscibility, are called subcritical extraction solvents, and the extraction process is called subcritical extraction. Solvents suitable for extraction and desolvation at room temperature or lower are defined as low-temperature subcritical solvents. Currently, the five main low-temperature subcritical solvents suitable for industrial production include propane, butane, dimethyl ether, tetrafluoroethane, and liquid ammonia. In short, low-temperature subcritical bioextraction is a low-temperature, low-pressure, and highly effective physical method.
Definition of a
low-temperature subcritical extraction solvent: The subcritical state of a solvent is defined as the state in which a solvent exists in a liquefied state under a certain pressure within a temperature range above its boiling point but below its critical temperature. This is also the liquefied state of a gas. In this state, leveraging its physical properties of miscibility, it is used as a solvent for extracting biocomponents. Currently, the five main low-temperature subcritical solvents suitable for industrial production include propane, butane, dimethyl ether, tetrafluoroethane, and liquid ammonia.

Many solvents with high boiling points, such as water, ethanol, and ether, have high subcritical temperatures, generally above the destruction temperature of certain heat-sensitive biological components. While we don't use them as low-temperature subcritical solvents, the unique extraction properties of their subcritical state can be utilized. These include increased permeability, changes in dielectric constant, reduced surface tension, and pH changes. Compared to similar solvents below their boiling point, these solvents offer advantages such as higher extraction efficiency and a wider range of extractable components. In other words, re-examining these conventional solvents through a subcritical lens can yield many unexpected new discoveries.
CO2 has a critical temperature of 31°C, a critical pressure of 7.15 MPa, and a critical density of 469 kg/m³. Therefore, supercritical CO2 extraction must be performed above 31°C. However, to enhance extraction performance, the CO2 fluid at the supercritical temperature is generally pressurized to 20 MPa or even 50 MPa to increase its density and, therefore, its ability to extract large molecules. The principle of subcritical extraction is that, under a certain pressure, a liquefied subcritical solvent is used to countercurrently extract the material. The solvent in the extract (liquid phase) is evaporated, vaporizing the solvent and separating it from the extracted target components to obtain the product. The extracted material then evaporates the adsorbed solvent, yielding another product (solid phase). The vaporized solvent is compressed, heat-exchanged, and liquefied for recycling. The entire low-temperature subcritical extraction process can be performed at room temperature or lower, thus minimizing damage to heat-sensitive components in the material. This is an advantage of low-temperature subcritical extraction.

Main advantages of low-temperature subcritical extraction:
① Excessive heating is not required during the extraction and desolventizing processes, minimizing damage to heat-sensitive components in the material and effectively preserving volatile components in the extracted product.
② The five solvents used and studied have different polarities, allowing for a wide range of target polarities to be extracted.
③ A certain degree of extraction selectivity is achieved.
④ Energy efficiency: evaporation of the extract consumes less energy, and the desolventizing process does not require high temperatures.
⑤ Compared to supercritical extraction, it requires less investment and production costs, making it easier to scale up.
Low-temperature subcritical fluid bioextraction technology, also known as DDG extraction technology, is based on its low temperature, low pressure, and high efficiency. With its high efficiency, environmental friendliness, and energy-saving characteristics, subcritical low-temperature extraction technology has shown broad application prospects in areas such as oil extraction and natural product separation. With the continuous development and improvement of this technology, we believe it will be applied and promoted in even more fields in the future.
![]() Service Coverage
Service Coverage
![]() FAQ
FAQ

 The subcritical state of a solvent is defined as the state in which it exists in a liquefied state under a certain pressure within a temperature range above its boiling point but below its critical point. Solvents used in this state, leveraging their physical properties of miscibility, are called subcritical extraction solvents, and the extraction process is called subcritical extraction. Solvents suitable for extraction and desolvation at room temperature or lower are defined as low-temperature subcritical solvents. Currently, the five main low-temperature subcritical solvents suitable for industrial production include propane, butane, dimethyl ether, tetrafluoroethane, and liquid ammonia. In short, low-temperature subcritical bioextraction is a low-temperature, low-pressure, and highly effective physical method.
The subcritical state of a solvent is defined as the state in which it exists in a liquefied state under a certain pressure within a temperature range above its boiling point but below its critical point. Solvents used in this state, leveraging their physical properties of miscibility, are called subcritical extraction solvents, and the extraction process is called subcritical extraction. Solvents suitable for extraction and desolvation at room temperature or lower are defined as low-temperature subcritical solvents. Currently, the five main low-temperature subcritical solvents suitable for industrial production include propane, butane, dimethyl ether, tetrafluoroethane, and liquid ammonia. In short, low-temperature subcritical bioextraction is a low-temperature, low-pressure, and highly effective physical method.